Answer: The reaction is the type of double displacement reaction.
Step-by-step explanation:
Double displacement reaction: It a type of chemical reaction in which , two reactants exchange their ions to give new products.Same pattern of chemical reaction is being followed in the given reaction.
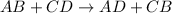
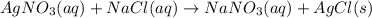
Hence, the correct answer is double displacement reaction.