The chemical formula represents the number of atoms of each element in a given molecule
a) water:

b) Ammonia:
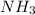
c) carbondioxide:

d) Flourine:
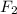
e) methane:
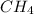
f) ethane:
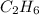
Structural formula is based on hybridization:
a) water: sp3 hybridized (two lone pair of electrons on central oxygen atom) shape "Bent"
b) Ammonia: sp3 hybridized (one lone pair of electrons on central oxygen atom) shape "pyramidal"
c) carbondioxide: linear
d) Flourine: linear
e) methane: sp3 hybridized , shape : tetrahedral
f) ethane: sp3 hybridized,sp3 hybridized (two lone pair of electrons on central oxygen atom) shape "Bent"
The structural formula and electron dot structures are shown in the figure.