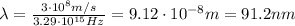1) Frequency:
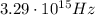
the energy of the photon absorbed must be equal to the ionization enegy of the atom, which is
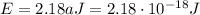
The energy of a photon is given by
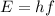
where
 is the Planck's constant. By using the energy written above and by re-arranging thsi formula, we can calculate the frequency of the photon:
is the Planck's constant. By using the energy written above and by re-arranging thsi formula, we can calculate the frequency of the photon:
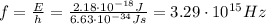
2) Wavelength: 91.2 nm
The wavelength of the photon can be found from its frequency, by using the following relationship:
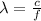
where
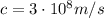 is the speed of light and f is the frequency. Substituting the frequency, we find
is the speed of light and f is the frequency. Substituting the frequency, we find