HI has the least volume
Further explanation
Given
Gasses and molar mass
Required
The least volume
Solution
Ideal gas law
PV=nRT
V = (nRT)/P
n = mass : MW(Molar weight=molar mass)
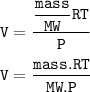
so V is inversely proportional to MW(molar mass)
Because the values of P, R, T, and mass are the same, what matters is the value of the molar mass of each compound. The larger the molar mass the smaller the volume
From the available molar mass data, HI has the largest molar mass, so HI has the smallest volume