Potassium chloride reacts with ammonium nitrate to give ammonium chloride and potassium nitrate.
This is a type of double displacement reaction. The balanced chemical equation can be represented as,

Total ionic equation for this reaction will be,
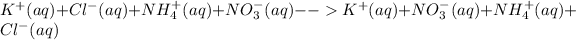
There is no apparent reaction as this reaction is not accompanied by the formation of a gas or a solid precipitate. We cannot observe any visual reaction as there is not net reaction taking place. All the ions remain as spectator ions.