Answer : 2 and 1 are the numbers in the place of X and Y in
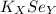 .
.
Explanation :
Crisscross method : It is a method of finding a chemical formula in which ionic bond is formed by combining of metal and non-metal.
The charge on Potassium ion is +1 and the charge on Selenide ion is -2.
The potassium ions combined with the selenide ions to form a Potassium selenide. Image is also show below.
According to the crisscross method,
The formula of Potassium selenide is
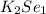 .
.
Therefore, 2 and 1 are the numbers in the place of X and Y in
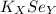 .
.