Answer:- 152.1 J
Solution:- This type of problems are solved by using the formula:
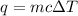
where, q is the heat energy, m is the mass, c is specific heat and
 is change in temperature.
is change in temperature.
mass of iron is given as 26 g. temperature is changing from 13 degree C to 26 degree C.
So,
 = 26 - 13 = 13 degree C
= 26 - 13 = 13 degree C
specific heat for iron is 0.450 Joule per gram per degree C.
let's plug in the values in the formula and do calculations for q.
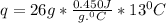
q = 152.1 J
So, 152.1 J of heat is absorbed by the iron skillet.