Answer:
a) 2.4 × 10²³ atoms Cu; b) 2.4 × 10²³ atoms Au; 2.4 × 10²³ molecules acetone
Step-by-step explanation:
There are Avogadro’s number of particles in one mole of those particles.
a) Cu
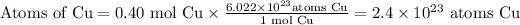
Thus, 0.40 mol of any particles contains 2.4 × 10²³ of those particles.
b) 0.40 mol Au = 2.4 × 10²³ atoms Au
c) 0.40 mol acetone = 2.4 × 10²³ molecules acetone