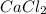Answer : The correct formula of the given compound will be,
Explanation :
Calcium chloride is an ionic compound because calcium element is a metal and chloride element is a non-metal. The bond formed between a metal and a non-metal is always ionic in nature.
The nomenclature of ionic compounds is given by:
1. Positive ion is written first.
2. The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
The charge on calcium is (+2) and the the charge on chloride is (-1). The charges are not balanced. Thus they combine and their charges are exchanged and written in simplest whole number ratios to give neutral
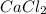 .
.
Hence, the formula of the compound calcium chloride will be