Answer:
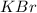
Explanation: An ionic bond is formed when an element completely transfers its valence electron to another element. The element which donates the electron forms cations and the element which accepts the electrons forms anions. This bond is formed between a metal and an non-metal.
Covalent bonds are formed by sharing of electrons between non metals.
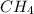 ,
,
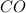 and
and
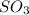 are all covalent compounds.
are all covalent compounds.
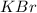 is an ionic compound formed by combination of
is an ionic compound formed by combination of
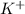 which is formed by loss of one electron by potassium and
which is formed by loss of one electron by potassium and
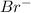 which is formed by gain of one electron by bromine.
which is formed by gain of one electron by bromine.