Answer: The reaction do take place and it leads to the formation of potassium chloride and iodine gas.
Step-by-step explanation:
Single displacement reaction is defined as the reaction in which more reactive element displaces a less reactive element from its chemical reaction.
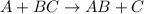
Element A is more reactive than element B.
The chemical equation for the reaction of elemental chlorine and potassium iodide follows:
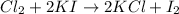
As, chlorine gas is more reactive than iodine. So, it will easily replace iodine from its chemical reaction.
Hence, the reaction do take place and it leads to the formation of potassium chloride and iodine gas.