Answer: Object B will heat up more.
Step-by-step explanation:
The formula for specific heat is as follows.
Q =
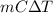
Where,
Q = heat provided
m = mass
C = specific heat
 = change in temperature
= change in temperature
Now, both the objects have same mass and equal amount of heat is applied.
According to the formula, the equation will be as follows.
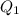 =
=
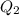
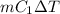 =
=
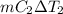
Cancel m from both sides, as mass is same. Therefore,
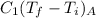 =
=
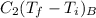
Cancel out the initial temperature and put the values of specific heat, then the equation will be as follows.
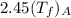 =
=
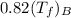
Therefore, from the above equation it can be concluded that the object with low specific heat will heat up more as its specific heat will be inversely proportional to its final temperature.
Hence, object B will heat up more.