Answer: Mass is neither lost nor gained during a chemical change.
Step-by-step explanation:
Antoine Lavoisier’s Law of Conservation of Mass:
'In a chemical reaction the mass can neither be created and nor be destroyed'.
In a chemical reaction the mass of reactants is equal to the mass of products which means that the mass remain conserved.
For Example: In a chemical reaction:
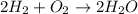
Mass of reactants = Mass of products
Mass of hydrogen molecule + mass of oxygen molecule = Mass of water
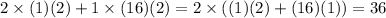
From this we can see that the mass remains conserved in the chemical reaction.