Answer:-
Solution:- As is clear from the given Ka value, Cinnamic acid is a weak acid. let's calculate the moles of acid and KOH added to it from their given molarities and mL.
For KOH,

= 0.002 mol
For Cinnamic acid,
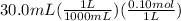
= 0.003 mol
Acid and base react as:
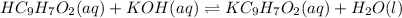
The reaction takes place in 1:1 mol ratio. Since the moles of acid are in excess, the acid is still remaining when all the kOH is used.
0.002 moles of KOH react with 0.002 moles of Cinnamic acid to form 0.002 moles of potassium cinnamate. Excess moles of Cinnamic acid = 0.003 - 0.002 = 0.001
As the solution have weak acid and it's salt(or we could say conjugate base), it is a buffer solution and the pH of the buffer solution could easily be calculated using Handerson equation:
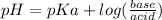
pKa could be caluted from given Ka value using the formula:
pKa = - log Ka
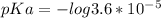
pKa = 4.44
let's plug in the values in Handerson equation and calculate the pH:
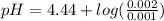
pH = 4.44+0.30
pH = 4.74
So, the first choice is correct, pH is 4.74.