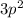Answer: The X represents
Step-by-step explanation:
We are given:
An element having 14 electrons
The distribution of electrons around the nucleus of an atom in defined orbits is defined as the electronic configuration of an atom.
The electronic configuration given to us is
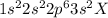
The next sub-shell that is filled after '3s' sub-shell is '3p' Till now, 12 electrons have been filled up. The remaining electrons are filled in '3p' sub-shell.
Now, the electronic configuration becomes:
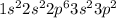
Hence, the X represents