Answer: For topic 2
Density of Iron = 8 g/mL
Density of Zinc = 7 g/mol
For topic 3
% error for Iron = 1.78%
% error for Zinc = 1.96%
Explanation:
For Topic 2
Density is calculated by using the formula:
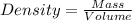
Here, 2 elements are given
For iron, Mass = 32.0 g
Volume = 4.0 mL
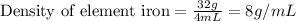
For Zinc, Mass = 42.0 g
Volume = 6.0mL
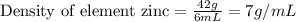
For Topic 3
% error is calculated by:
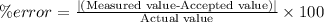
For iron,
Actual density of iron = 7.86 g/mL
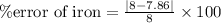
% error of iron = 1.78 %
For Zinc,
Actual density of Zinc = 7.14 g/mL
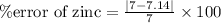
% error of iron = 1.98 %