Answer: For question 1, correct option is D.
For question 2, correct option is B.
Explanation:
For question 1: A polar covalent bond is the chemical bond where electrons are unequally shared between the two different elements having different electrongativities. 2 poles are generated in this bond.
According to Pauling's electronegativity rule, difference between the electronegativities of elements in a polar covalent bond is from 0.5 to 2.0
Hence, polar covalent bond can never exist between the atoms of the same element.
For Question 2:
Option 1: Inhibitor decreases the rate of the reaction, but we are removing the inhibitor, so the rate will be increased.
Option 2: For a reaction,
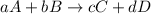
![rate=k[A]^a[B]^b](https://img.qammunity.org/2019/formulas/chemistry/high-school/uoe7lbxw5bvc5s4i7pwyb5gdo0nosyq5t2.png)
As rate is directly proportional to the reactants, so by lowering the concentration of the reactants, the rate will also decrease.
Option 3: By increasing the temperature, collisions also increases and this collision results in the formation of the product. Hence, increasing the rate of the reaction.
Option 4: Adding a catalyst increases the rate of the reaction by lowering down the activation energy of the reactants.