Answer: The average atomic mass of this element is 428.68 .
Step-by-step explanation:
Average atomic mass =
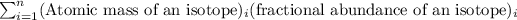
Atomic mass of Swidorskium-428 = 428
Abundance of Swidorskium-428 = 86.4%=0.864
Atomic mass of Swidorskium-433. = 433
Abundance of Swidorskium-433 = 13.6= 0.136
The atomic mass of the element is
= (atomic mass of Swidorskium-428 )(abundance) + (atomic mass of Swidorskium-433)(abundance)
The atomic mass of Swidorskium = (428)(0.864)+(433)(0.136) = 428.68