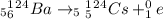Answer:- 0/+1e
Solution:- For the nuclear equations, the sum of mass numbers of reactants must be equal to the products. Similarly, the sum of atomic numbers of reactants must be equal to the products.
For the given reaction, The atomic number for reactant side is 56 and for the product side it is 55. To make them equal, the atomic number of the unknown product must be 1.
Mass number of the reactant side is 124 and the product side is also 124. Since it's already equal, the mass number of the unknown must be 0.
Since there is a decrease in atomic number by one unit, it is +beta decay reaction. beta particle is positron which is positively charged electron. So, the right choice is 0/+1e and the equation is written as: