Answer: The average atomic mass of lonacapium 82.1581 amu.
Solution: To calculate average atomic mass of element, we use the formula:
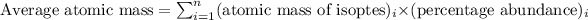 ....(1)
....(1)
First isotope, Lc-82
Atomic Mass of Lc-82 isotope=81.2372 amu
Percentage abundance = 47.36%= 0.4736
Second isotope, Lc-83
Atomic Mass of Lc-83 isotope=82.5759 amu
Percentage abundance = 38%= 0.3800
Third isotope, Lc-85
Atomic Mass of Lc-85 isotope =84.0536 amu
Percentage abundance = 100% - 47.36% -38% = 14.64%= 0.1464
Putting the values in equation 1.
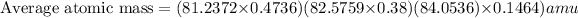
Average atomic mass of Loncapium = 82.1581 amu