Answer:
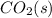 represent solid form of carbon-dioxide gas in the reaction.
represent solid form of carbon-dioxide gas in the reaction.
Step-by-step explanation:
In the given reaction solid phase of carbon-dioxide is changing directly into gaseous phase of carbon-dioxide. This process of changing of substance from solid to gaseous state directly by escaping the liquid state is known as Sublimation.
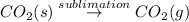
Here
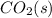 is representing the solid state of carbon-dioxide which is also known as Dry-ice.
is representing the solid state of carbon-dioxide which is also known as Dry-ice.