Answer: Option (d) is the correct answer.
Step-by-step explanation:
According to law of conservation of mass, mass can neither be created nor it can be destroyed as it can only be transferred from one form to another.
This means that in a chemical reaction the mass of reactants is equal to the mass of products.
For example,
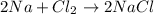
Total mass of reactants is as follows.
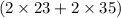 g/mol
g/mol
= 116 g/mol
Total mass of products is as follows.
2NaCl =
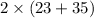
= (2 \times 58) g/mol
= 116 g/mol
Hence, we can conclude that mass is conserved in both physical and chemical changes.