1. Salts of a weak acid and a strong base produce solutions that are basic.
That’s because the salt of a weak acid hydrolyzes in water to form hydroxide ions.
A- + H₂O ⇌ HA + OH⁻
2. The common ion effect is the phenomenon in which the addition of a charged particle common to two solutes decreases the solute concentration.
AgCl(s) ⇌ Ag⁺(aq) + Cl⁻(aq)
The addition of NaCl reduces the concentration of Ag⁺ because the added common ion (Cl⁻) forces the position of equilibrium to shift to the left.
3. When hydrogen peroxide decomposes naturally, the products are oxygen and water.
2H₂O₂ ⟶O₂ + 2H₂O
4. An oxidizing agent is the substance that is reduced.
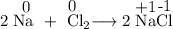
The Na is oxidized, so the Cl atoms in Cl₂ are the oxidizing agent.
The oxidation number of Cl goes from 0 to -1, so Cl is reduced.