Answer : The excess reactant in the combustion of methane in opem atmosphere is
 molecule.
molecule.
Solution : Given,
Mass of methane = 23 g
Molar mass of methane = 16.04 g/mole
The Net balanced chemical reaction for combustion of methane is,
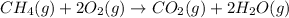
First we have to calculate the moles of methane.
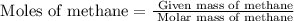 =
=
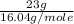 = 1.434 moles
= 1.434 moles
From the above chemical reaction, we conclude that
1 mole of methane react with the 2 moles of oxygen
and 1.434 moles of methane react to give
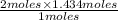 moles of oxygen
moles of oxygen
The Moles of oxygen = 2.868 moles
Now we conclude that the moles of oxygen are more than the moles of methane.
Therefore, the excess reactant in the combustion of methane in open atmosphere is
 molecule.
molecule.