Volume of Fe(NO₃)₃ = 5.00 ml
Total voluem of the solution = 20.0 ml
Concentration of Fe(NO₃)₃ = 0.002 M
Assuming complete dissociation of Fe(NO₃)₃ concentration of Fe³⁺ = 0.200 M
Using the dilution equation, M₁V₁ = M₂V₂
where M₁ and M₂ are the initial and final concentration of Fe³⁺ respectively and V₁ and V₂ are the initial and final volume of Fe³⁺ respectively
Plugging the data we get,
M₂ =
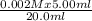 = 5 x 10⁻⁴
= 5 x 10⁻⁴
Therefore, the concentration of Fe³⁺ once diluted in the mixture is 5 x 10⁻⁴M