Answer:- 0.0002 ppm
Solution:- 1 ppm is one mg of solute per liter of solvent or one kg of solvent.
It asks to convert
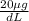 pb in blood to ppm. density of blood is
pb in blood to ppm. density of blood is
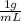 .
.
Let's convert micrograms to milligrams and also calculate the mass of blood from it's given density. The set up could be made using dimensional analysis and doing unit conversions as:
First the set up for the conversion of micrograms of Pb to milligrams:
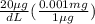
=
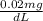
Now, the set up for converting dL of blood to kg using it's given density and unit conversion from dL to mL as:
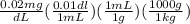
= 0.200 ppm