Number of protons is same as the atomic number and number of neutrons is equal to the subtraction of the atomic number from the mass number i.e.
number of neutrons = atomic number - mass number
Now, the atomic number =
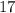 = number of protons
= number of protons
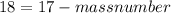
mass number =
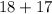
=
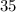
Thus, the element is chlorine.
Name of the element is Chlorine-35.