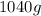The balanced chemical reaction is given as:
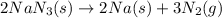
Now, convert
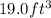 into litres.
into litres.
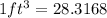
So,
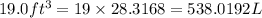
Density is equal to the ratio of mass to the volume.

where, M = mass and V= volume
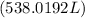
Substitute the value of density and volume in formula to get the value of mass.
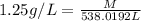
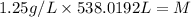
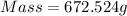
Now, number of moles of
 gas=
gas=
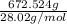
=
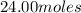
According to the reaction, 2 moles of sodium azide gives 3 moles of nitrogen gas.
Now, in 24.00 moles of nitrogen gas produced from=
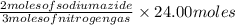 of nitrogen gas, moles of sodium azide.
of nitrogen gas, moles of sodium azide.
number of moles of sodium azide =
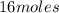
Mass of sodium azide in g =
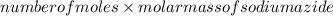 .
.
=
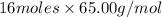
=
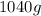
Thus, mass of sodium azide which is required to produce
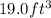 of nitrogen gas =
of nitrogen gas =