Mass of lead (II) chromate is 51 g. The molecular formula is
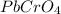 and its molar mass is 323.2 g/mol
and its molar mass is 323.2 g/mol
Number of moles can be calculated using the following formula:
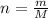
Here, m is mass and M is molar mass.
Putting the values,
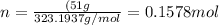
Therefore, number of moles of lead (II) chromate will be 0.1578 mol.