The reaction between hydrogen and oxygen to form water is given as:
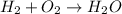
The balanced reaction is:
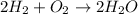
According to the balanced reaction,
4 g of hydrogen (
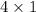 ) reacts with 32 g of oxygen (
) reacts with 32 g of oxygen (
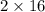 ).
).
So, oxygen reacted with 29.4 g of hydrogen is:
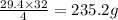
Hence, the mass of oxygen that is reacted with 29.4 g of hydrogen is 235.2 g.