Answer:
A.
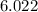 x
x
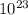
Step-by-step explanation:
The number of particles in one mole is known as Avogadro's Number. This number is a constant that is based the number of atoms in a sample of Carbon-12. Avogadro's Number is so large that it is always written in scientific notation, which is why the constant is written as 2 products as opposed to 1 number. Moles and Avogadro's number are useful in Stoichiometry or when determining the formula units or mass of a sample.