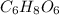Number of moles is defined as the ratio of given mass in g to the molar mass.
First, convert the given mass of carbon dioxide in mg to g:
1 mg = 0.001 g
17.61 mg = 0.01761 g
Number of moles of carbon dioxide =
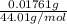
=
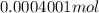
Mass of carbon = number of moles of carbon dioxide \times molar mass of carbon
=
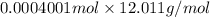
=
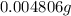
Number of moles of water=
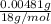
=

Since, water contains two hydrogen atoms. Thus,
Moles of hydrogen =
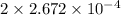
=
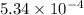
Mass of hydrogen =
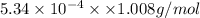
=
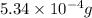
Mass of oxygen =
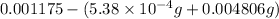
=
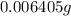
Number of moles of oxygen =
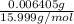
=
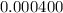
Now,
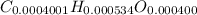
Divide the smallest number to get the whole number,
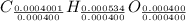
we get,
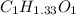
Now, multiply all the subscript by 3 to get the whole number,
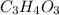 (empirical fomula)
(empirical fomula)
Molar mass of the compound =
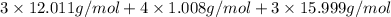
=
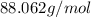
Divide given molar mass of the compound with the molar mass of the compound.
=
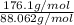
=
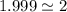
Thus, multiply the subscripts of empirical formula by 2 to get the molecular formula, we get:
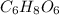
Hence, empirical formula is
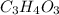 and molecular formula is
and molecular formula is