A molecular formula represents the exact number of atoms present for each element in the compound.
For carbon atom:
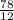 =
=
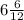 ( as molar mass of carbon is 12 g/mol)
( as molar mass of carbon is 12 g/mol)
Now, 6 carbon atoms are present and rest are hydrogen atoms i.e. 6
Thus, formula becomes
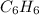 (
(
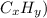
Now, check for unsaturation:
Degree of unsaturation =
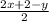
Substitute the value of x and y,
Degree of unsaturation =
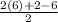
=
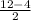
=
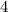 implies one ring and three double bonds.
implies one ring and three double bonds.
Thus, formula comes out to be
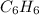 i.e. benzene ring.
i.e. benzene ring.