Since, hydrogen atom consist of one proton and an oxygen atom consist of eight protons.
Now, density of water is 1 g/ml
Given volume =
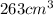 = 263 mL
= 263 mL
So, weight of water = 263 g
Number of moles of water =
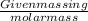
=
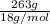
= 14.61 g/mol
Number of protons =
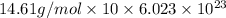
=
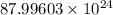
As, charge on one proton =
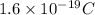
Thus, total charge =
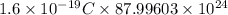
=
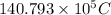
=
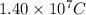
Thus,
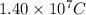 is the total number of coulombs of positive charge.
is the total number of coulombs of positive charge.