Answer:- 0.59 mL
Solution:- From given information, 580 mg of the drug are present in 1.8 mL of solution. It asks to calculate the mL of solution that would contain 190 mg of the dug.
This could easily be solved using ratios as:

On rearranging:
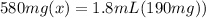
divide both sides by 580mg.

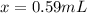
So, 0.59 mL of the solution contains 190 mg of the drug.