Formula used for determining the number of moles:
number of moles =
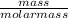 -(1)
-(1)
Mass of
 = 14.41 mg (given)
= 14.41 mg (given)
Mass of
 = 3.93 mg (given)
= 3.93 mg (given)
Number of moles of
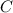 from
from
 =
=
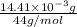
=
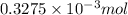
Number of moles of
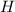 from
from
 =
=
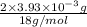
=
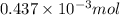
Mass of carbon =
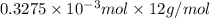
=
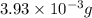
Mass of hydrogen =
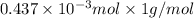
=
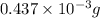
Mass of oxygen = total mass of the compound - mass of carbon- mass of hydrogen
=
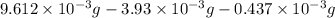
=
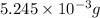
Now, number of moles of oxygen =
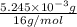
=
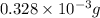
To identify the empirical formula; divide the number of moles of carbon, hydrogen and oxygen with least number of moles.
Thus,
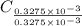
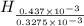
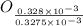
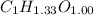
Now, multiply the numbers with 3 to get the hydrogen number in whole number, we get
The empirical formula =
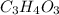
The empirical weight =
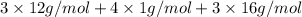
=

Divide the molecular weight with empirical weight to find the factor between empirical formula and molecular formula , we get
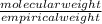 =
=
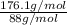
=
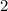
Now, multiply the numbers of carbon, hydrogen and oxygen with 2 to get molecular formula, we get
Number of carbon = 6
Number of hydrogen = 8
Number of oxygen = 6
Thus, molecular formula is
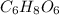 .
.