Answer : The molarity of Cl- ions is 0.00461M
Explanation :
Step 1 : Find moles of NiCl₂.6H₂O.
The moles of a substance are calculated as
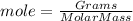
The molar mass of NiCl₂.6H₂O is calculated as follows.
Ni + 2Cl + 12H + O = 58.69 + 2(35.45) +12 (1.01) + 6(16) = 237.71 g/mol
Moles of NiCl₂.6H₂O =
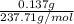
Moles of NiCl₂.6H₂O = 0.000576
Step 2 : Use mole ratio to find moles of Cl
There are 2 moles of Cl in 1 mol of NiCl₂.6H₂O.
Therefore the mole ratio of NiCl₂.6H₂O and Cl- is 1:2
The moles of Cl- = 2 x 0.000576 = 0.00115
Step 3 : Find molarity of Cl
The molarity of Cl- can be calculated as,
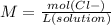
The final volume of the solution is 250 mL = 0.25 L.
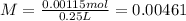
The molarity of Cl- ions is 0.00461M