We have the value of
Total energy produced in the chemical reaction=653 550 KJ
Time needed=142.3min
To calculate the rate of energy transfer, that is the amount of energy produced per minute.
Rate of energy transfer=
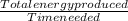
=
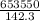
=4592.76 KJ min⁻¹
So, the rate of energy transfer is 4592.76 KJ min⁻¹.