Answer:- Volume will be 15 mL.
Solution:- If we look at the given information then it is Boyle's law as the temperature is constant and the volume changes inversely as the pressure changes.
The equation used for solving this type of problems is:
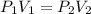
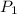 = 3.0 atm
= 3.0 atm
 = 5.0 mL
= 5.0 mL
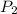 = 1.0 atm
= 1.0 atm
 = ?
= ?
Let's plug in the values in the equation:
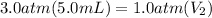
 = 15 mL
= 15 mL
So, the volume of the air bubble at the surface will be 15 mL.