Answer:
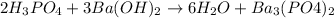
Step-by-step explanation:
Hello,
In this case, it is necessary to remember that a neutralization chemical reaction occurs when an acid reacts with a hydroxide to yield a salt and water. In such a way, the only reaction matching that definition is:
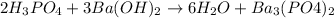
As phosphoric acid reacts with barium hydroxide to yield a neutral salt, barium phosphate and water.
Regards.