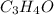Asnwer : Empirical formula of a compound is :
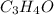
Given information : C = 64.3 % , H = 7.2 % , O = 28.5 %
Step 1 : Convert the given percentage (%) to grams.
Explanation : Let the total mass of the compound be 100 grams.
Mass of C = 64.3 g
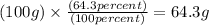
Mass of H = 7.2 g
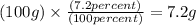
Mass of O = 28.5 g
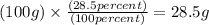
Step 2 : Convert the grams of each compound to moles.
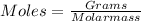
Molar mass of C = 12.0g/mol
Molar mass of H = 1.0 g/mol
Molar mass of O = 16.0g/mol
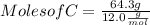
Moles of C = 5.36 mol
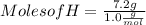
Moles of H = 7.2 mol
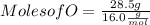
Moles of O = 1.78 mol
Step 3 : Find the mole ratio of C , H and O
Mole ratio is calculated by dividing the mole values by the smallest value.
Mole of C = 5.36 mol , Mole of H = 7.2 mol , Mol of O = 1.78 mol
Out of the three mole values , mole value of O that is 1.78 mol is less , so we divide all the mole values by 1.78 mol.
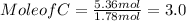
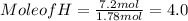
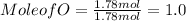
C : H : O = 3 : 4 : 1
So empirical formula of the compound is
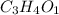 or
or