Acid : A species (molecule or ion) that can lose a proton (H+) is called an acid.
Conjugate base : A conjugate base is simply an acid that has given up a proton(H+).
1.
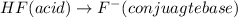
HF is an acid which will lose its proton(H+) and form its conjugate base
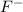
2.
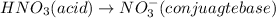
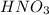 is an acid which will lose its proton(H+) and form its conjugate base
is an acid which will lose its proton(H+) and form its conjugate base
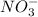
3.
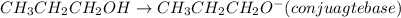
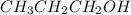 will lose its proton(H+) and form its conjugate base
will lose its proton(H+) and form its conjugate base
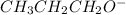
Note : An acid when loses its proton(H+) form the conjugate base.