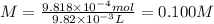Answer:
0.100 M
Step-by-step explanation:
KHP is used to standardize the NaOH solution, according to the following neutralization reaction.
KC₈H₅O₄ + NaOH → NaKC₈H₄O₄ + H₂O
We can establish the following relations.
- The molar mass of KHP is 204.22 g/mol.
- The molar ratio of KHP to NaOH is 1:1.
The moles of NaOH that reacted with 0.2005 g of KHP are:
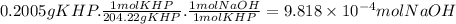
The molarity of NaOH is: