Answer : The mass of
 are, 79.845 grams
are, 79.845 grams
In the given reaction, the coefficient for calcium oxide is, 1
Explanation :
Part 1 : Given,
Moles of
 = 0.500 mole
= 0.500 mole
Molar mass of
 = 159.69 g/mole
= 159.69 g/mole
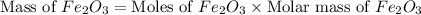
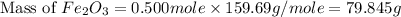
Therefore, the mass of
 are, 79.845 grams
are, 79.845 grams
Part 2 :
The given balanced chemical reaction will be,
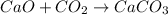
This reaction is a balanced reaction in which 1 mole of calcium oxide react with 1 mole of carbon dioxide to give 1 mole of calcium carbonate as a product.
Hence, the coefficient for calcium oxide is, 1