So we know that the molar ration of carbon dioxide to sugar is 6:1 in the balanced equation, so we must first find how many moles of carbon dioxide 200g is.
The molar weight of carbon dioxide is 44.008g - found from the atomic weights of the elements on the periodic table.
So we must solve for moles:
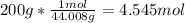
So then we can set up a proportion to solve for how many moles of sugar were produced:
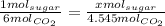
Then we solve for x:
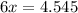
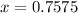
So now we know that 0.7575 moles of sugar were produced. To convert to grams, we must know the molar weight of sugar:
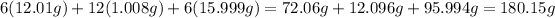
So then we can solve for grams of sugar:
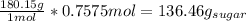
Therefore we know you can make 136.46 grams of sugar when using 200 grams of carbon dioxide.