According to Arrhenius theory, acid is a substance that releases H⁺ ions when dissolved in water.
In order to apply this theory, the substance must be soluble in water.
H₂SO₄ is highly soluble in water. It undergoes following dissociation reaction when dissolved in water.
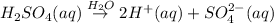
From the above equation, we can see that H₂SO₄ forms 2 H⁺ ions when dissolved in water. Therefore it behaves as an acid according to Arrhenius theory.