Given the volume of water sample = 2.00 L
Mass of lead = 150.0 μg
When we say that a solution is 1 ppm in concentration, it means that there is one part of solute per million parts of the solution.
One ppm = 1 mg solute/L solution
The concentration of lead in ppm:
150.0 μg ×
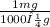 ×
×
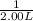
= 0.075 ppm