Answer: -
Zero
Explanation: -
Number of moles of sulphur S = 5 mol
Number of moles of O2 = 10 mol
The reaction between them will be synthesis type.
The product formed will be SO2.
The balanced chemical equation for this chemical reaction is
S + O2 -- > SO2
From the balanced equation we see that
1 mol of S reacts with 1 mol of O2
So, 5 mol of S will react with
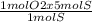
= 5 mol of O2.
O2 left = 10 – 5 = 5 mol
Sulphur is limiting reagent and will be used up