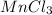We use the given masses of the reactants to calculate the moles of Mn and Cl. Empirical formula represents the simplest mole ratio of atoms present in a compound.
Moles of Mn =
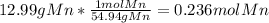
Moles of Cl =
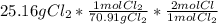 = 0.710 mol Cl
= 0.710 mol Cl
Simplest mole ratio:
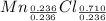
So the empirical formula is