As, volume is constant here, we can apply Gay-Lussac's law. It states that the pressure exerted on the sides of a container by an ideal gas is directly proportional to its absolute temperature for a given mass and constant volume. That is,
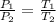
We have, P₁ = 41 psi
T₁= 15°C=15+273.15 = 288.15 K
T₂=32°C= 32+273.15 = 305.15 K
Substituting values,
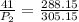
P₂=
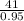
P₂=43.15 psi
The pressure in the tire would rise to 43.15 psi if it warms to a temperature of 32.0° C.